
Ozone is present in the environment both as a protective layer against UV-radiation in the stratosphere and as ground level ozone. The word ozone is often associated with the environmental threats: depletion of the ozone layer and increasing of the ground level ozone. The fact is that ozone is also used for disinfection of air, water and for a lot of other industrial processes such as bleaching of paper pulp. Ozone is a very reactive molecule that has a very strong oxidizing ability. It is this oxidizing ability that makes ozone such a potent disinfection method. The market for ozone is big for example in the USA and it is growing all over the world. This makes it important to be aware of what ozone is and its potentials.[su_spacer]
OZONE
Ozone is a molecule that is made up of three oxygen atoms i.e. O3 which is present in mother earths own natural cycle. In the Stratosphere, 10–50 km above our heads the sun is constantly changing a part of the airs oxygen molecules (O2) to ozone. The natural ozone layer that is created in that way has the ability to absorb the UV-light from the sun, more specific the UV-B radiation (280-315 nm). This ozone layer makes it possible for life to exist on the earth. Ozone can be produced in generators through using either UV-light or Corona effects. Such generators use dried air or oxygen as their source gas. By adding high voltage, the oxygen molecule splits to two oxygen atoms. These atoms can then combine with other oxygen molecules which make a molecule with three oxygen atoms, i.e. ozone.[su_spacer size=”10″]
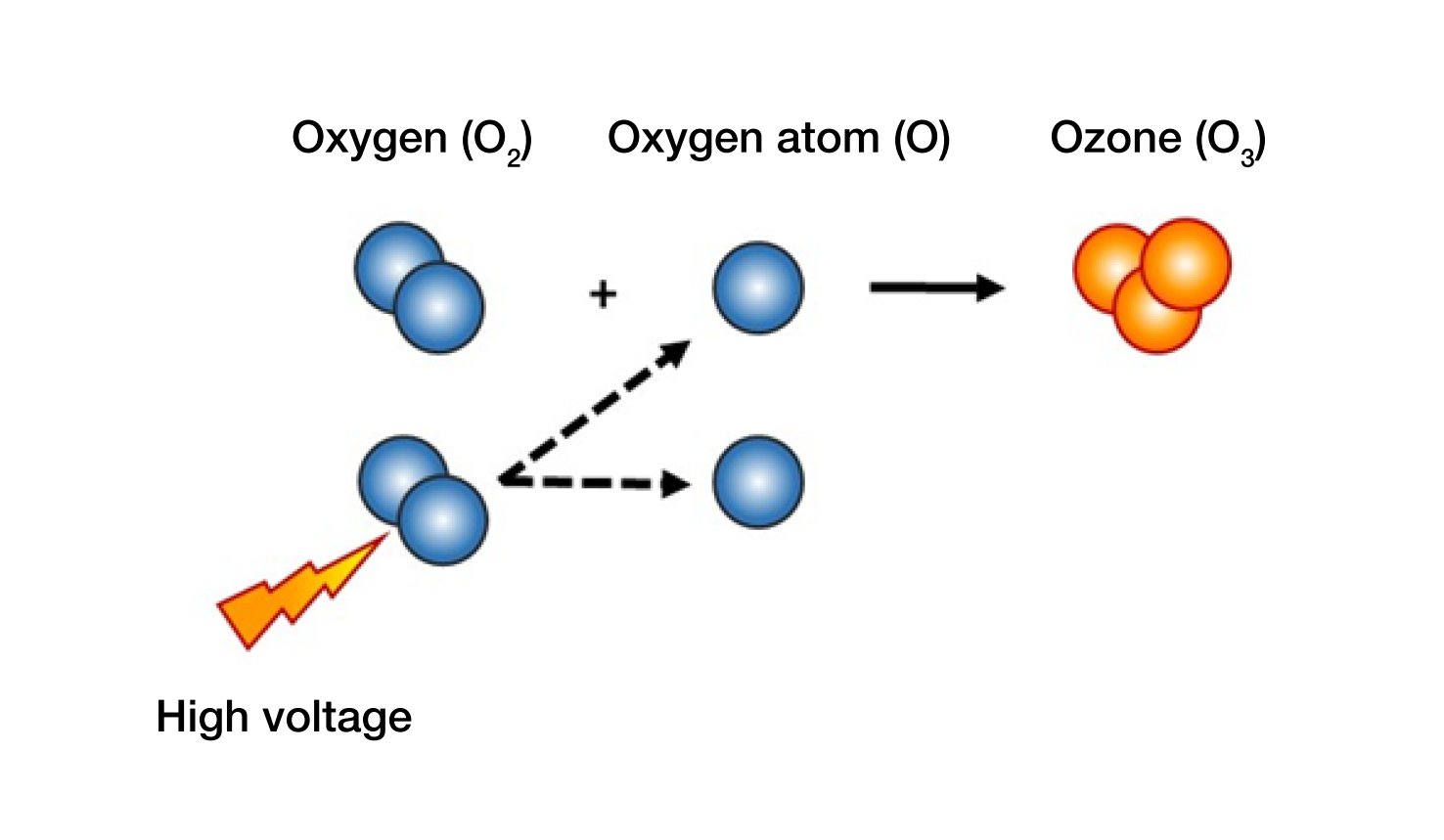 [su_spacer size=”10″]
[su_spacer size=”10″]
The ozone molecule has a strong oxidation potential which means that it reacts easily with other molecules and decomposes/transforms them. The ozone molecule is an instable molecule which means that, if it doesn’t collide with another oxidizeable molecule, it decomposes by itself to form oxygen again. The life-span of ozone ranges from a couple of minutes to an hour depending on the surrounding conditions (temperature, pressure, pollutions etc).[su_spacer]
INDUSTRIAl OZONE – GROUND-LEVEL OZONE – STRATOSPHERIC OZONE
It is important to differentiate between the ozone we produce industrially, ground-level ozone and stratospheric ozone. Stratospheric ozone is the ozone that is formed in the atmosphere with the rays from the sun as source of energy. When we speak of ozone depletion and ozone holes it means that the ozone up in the stratosphere produced by the sun in some way has disappeared. The biggest cause for this is the use of substances like Freon’s. The substances get oxidized by ozone when they reach the atmosphere. The formation of ground-level ozone is a partly natural process. Ozone is formed in strong electrical discharges from thunders and by photochemical reactions from pinewoods. The increasing amount of ground-level ozone is a result from the interaction between the sunlight and gases such as NOx and VOC (Volatile Organic Compounds).
Both nitrogen oxides and carbon oxides are naturally present in the atmosphere, just as the ozone is. The level of the nitrogen oxides and carbon oxides though have increased in recent times. This has also increased the level of ozone at ground level. In Europe it has increased about two times since the 19th century and in 1970 it was still increasing with about 1% per year. Nowadays, the increase seems to have stopped.
The industrial ozone is the ozone produced in ozone generators. This ozone is generated for use in some kind of application, for example cleaning water or air, or the bleaching of paper pulp. The ozone generated industrially will not come in contact with the surrounding. There are a number of rules and regulations that protect the environment from being exposed to ozone. In industrial processes, the excess ozone is passed through a catalytic converter where the ozone is changed back into oxygen. In this process, the energy of formation is released as heat. The catalytic conversion can be done in different ways, destruction with
manganese dioxide, thermically or activated carbon. UV-light is also an option in some applications.
Because ozone is an unstable molecule and decomposes spontaneously, it has to be generated on site. This means that you avoid any problems with associated with storage and transportation of gases. It is Important to know that when ozone is used in dissolved form, its concentration in the gas phase can be up to 200 000 ppm. When the ozone dissolves in the water the concentration is reduced to a few ppms. When the dissolved ozone later partly goes back to the air phase, it is rare to measure concentrations above 0.1 ppm in the air. After a short while it is not possible to detect any residuals of ozone in the water at all. This is a big difference compared to other chemicals such as chlorine. This is one reason why ozone should be seen as a more environmentally friendly alternative to these kinds of applications. The idea is to treat ozone with respect, but with an enhanced consciousness of what ozone is and its potentials, it is often a better alternative than chemicals.[su_spacer]
PROPERTIES OF OZONE
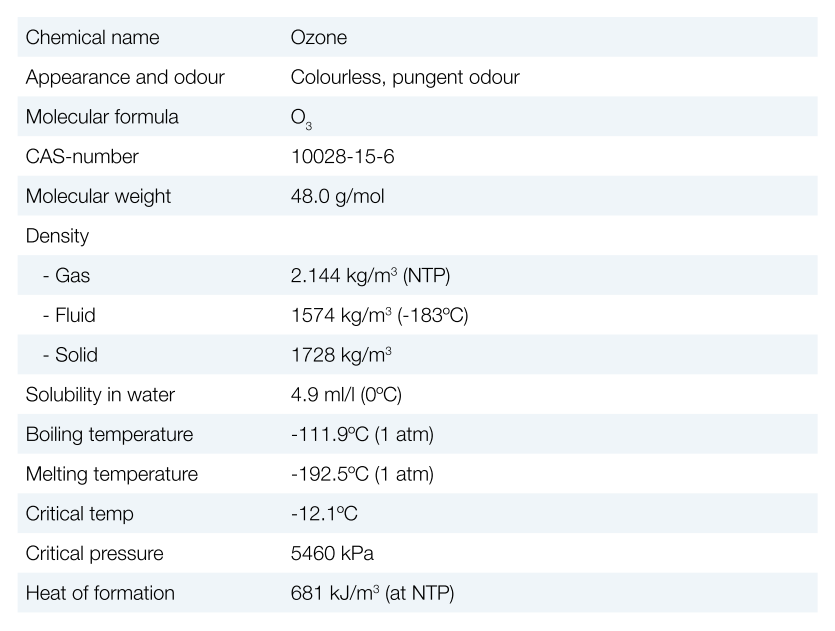 [su_spacer]
[su_spacer]
LIMITS AND RUlES
In 1997, ozone got GRAS status (Generally Recognized as Safe) from the FDA (Food and Drug Administration) in USA for use in the food industry. The classification means that ozone is allowed to be used as a sanitation agent and a disinfectant when used in amounts and applications according to GMP (Good Manufacturing Practises). GMP is today used by many food and pharmaceutical industries to make sure that rules and laws relating to the environment, humans and quality are followed.
Ozone had been used in the food-industry before it got the GRAS status. The reason for this is that advantages can easily be seen compared to for example chlorine. The ozone doesn’t result in any dangerous by-products which can occur in chlorine use.
When it comes to ozone, regulations for the emission rates are set by the health and drug administration. The limit for long-time exposure is set to 0.1ppm and the short-time exposure is set to 0.3ppm. The long-time exposure is referred to exposure during 8-hour workday, 5 days/week and the short-time exposure is set according to 15 min exposure.[su_spacer]
HEALTH EFFECTS
Ozone is a powerful oxidant which means that one should have respect for ozone but not be frightened of it. As with other oxidizing agents (for example chlorine and hydrogen peroxide), ozone is toxic above certain limits. Ozone is naturally present in our environment in concentrations around 0.01-0.15 ppm (~0.3 mg/m3) and in urban areas up to 1.0 ppm comparatively, copy machines, engines and transformers produces ozone in contents near 0.5ppm.
An advantage with ozone is its characteristic odour. You can always smell ozone far before the concentration is anyway near the limit of 0.1 ppm. This means that you are always aware that you are staying in an environment with increased ozone concentrations and thereby it is easy to avoid.
Symptoms when exposed to ozone are:
0.1 – 1 ppm Headache, irritation in eyes and throat
1-100 ppm Asthmatic symptoms, lack of appetite
If exposed to a higher dose of ozone during a shorter period the effects are irritation of the throat and coughing. These are not chronic problems and they will disappear within a couple of hours. Ozone has been used commercially for over 100 years and no deaths have been reported due to exposure to ozone.
When using ozone you should be aware of the emission regulations that are present. An easy way to make sure that the concentrations aren’t too high is to install an ambient ozone sensor. The sensor often has a set point that can give out an alarm when the concentrations are too high.